Why This Microcap Biotech Stock Surged 1,378% in a Single Day
FDA Breakthrough Therapy status sends Spruce Biosciences soaring as investors bet on a first-of-its-kind treatment for Sanfilippo syndrome.

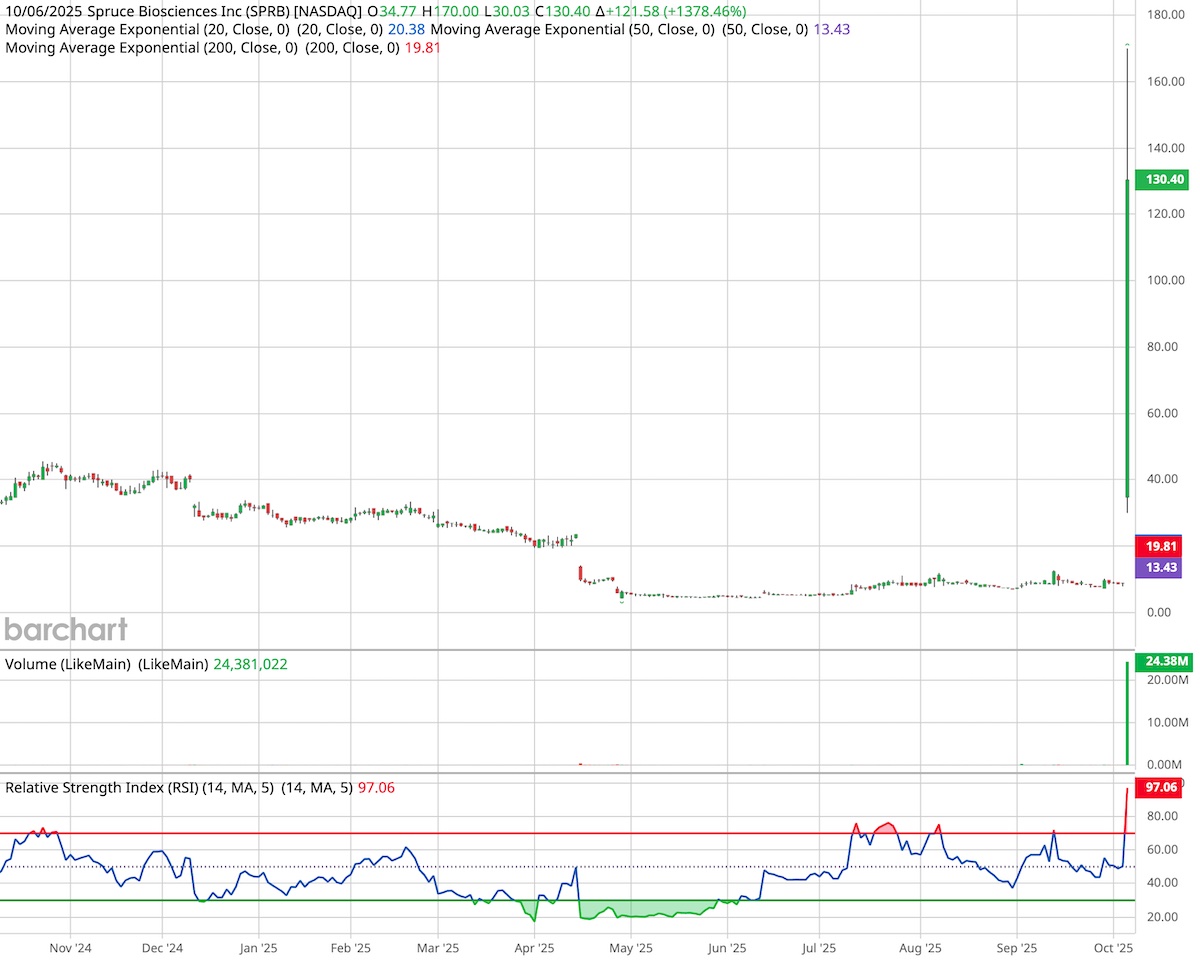
Shares of Spruce Biosciences (NASDAQ: SPRB) surged 1,378% to $130.40 on Monday after the U.S. Food and Drug Administration granted Breakthrough Therapy Designation (BTD) to tralesinidase alfa (TA-ERT) for Sanfilippo syndrome type B (MPS IIIB).
The announcement lit a fire under SPRB stock, which climbed by as much as 1,827% reaching an intra-day high of $170. Market dynamics amplified the reaction. Spruce recently regained its Nasdaq listing after a reverse split, shrinking its tradable float. When the FDA headline hit, demand overwhelmed supply, driving volume up more than 300,000% above average, from 7,981 to 24.17 million shares.
Also Read: Why Big Investors Who Can’t Buy Crypto Are Gobbling Up These Stocks Instead
The Catalyst
BTD delivers intensified FDA guidance, eligibility for rolling submission, and potential priority review. Critically, the agency confirmed that normalization of cerebrospinal-fluid heparan sulfate non-reducing end (CSF HS-NRE) is a surrogate biomarker “reasonably likely to predict clinical benefit,” opening an accelerated-approval path.
Integrated data across three studies over as many as five years showed TA-ERT normalized CSF HS-NRE and indicated stabilization of brain volume and cognition in children with MPS IIIB, a fatal neurodegenerative disorder with no approved therapies and an estimated U.S. prevalence below 1 in 200,000.
The Therapy
TA-ERT targets the missing NAGLU enzyme that causes toxic heparan sulfate buildup in the brain. The therapy fuses recombinant NAGLU to an IGF-2 peptide to improve cellular uptake via the mannose-6-phosphate pathway and is administered directly into the central nervous system. By restoring enzyme activity and clearing storage material, the therapy aims to modify the course of the disease rather than simply treat symptoms.
Also Read: How to Capitalize on the Five Pillars of Growth in the Red-Hot Wearables Market
Timeline
Spruce reaffirmed its near-term regulatory plan. With BTD in hand, the company can begin a rolling submission of its BLA and engage the FDA more frequently, potentially shortening the approval timeline once the package is complete.
Javier Szwarcberg, M.D., M.P.H., CEO of Spruce Biosciences, stated:
“We are pleased to receive U.S. FDA Breakthrough Therapy Designation as we prepare to submit the Biologics License Application of TA-ERT for the treatment of MPS IIIB in the first quarter of 2026. This designation highlights TA-ERT’s potentially transformative clinical impact as the first disease-modifying therapy to treat MPS IIIB in children impacted by this devastating condition.”
Risks and Outlook
Key next steps include final FDA alignment on the accelerated-approval plan, maintaining manufacturing readiness for a CNS-delivered biologic, and continued monitoring of long-term safety data. The therapy already holds Orphan, Fast Track, and Rare Pediatric Disease designations, which could yield a valuable Priority Review Voucher if approved.
Financially, Spruce remains a high-risk play. The company ended the second quarter with limited cash and will likely seek additional capital or a partnership to bridge the BLA submission and potential commercialization. Any equity raise could pressure shares even as it extends the runway.
Despite those risks, Monday’s record surge wasn’t a speculative anomaly; it reflected a genuine regulatory inflection point. The FDA’s Breakthrough designation and biomarker confirmation materially improved TA-ERT’s route to market. If Spruce keeps its 2026 filing on schedule and secures the financing to reach approval, the company could deliver the first disease-modifying therapy for Sanfilippo syndrome type B, an outcome that would permanently change both its valuation and its place in biotech.
Small Cap News Movers & Winner Deep Dive – By WealthyVC.com
We scan over 10,000 publicly listed stocks across all seven North American exchanges to uncover the market-moving news that actually matters—focusing on high-quality, liquid, growth-oriented companies in sectors attracting serious capital, like AI, blockchain, biotech, and consumer tech.
Each week, we publish Small Cap News Movers, a curated roundup of small and micro-cap stocks surging on meaningful catalysts. We break down what’s driving the move, tap into rumors swirling on social media, and surface sharp insights from both industry experts and retail sleuths.
From this list, we select one standout stock for our Small Cap Winner Deep Dive, released the next day, where we take a closer look at the fundamentals, narrative, and technicals that suggest this winner could keep running.
Powered by our proprietary 4-element, AI-driven analysis system, our goal is simple: cut through the noise, remove the emotion, and help investors dominate the small-cap market with momentum-driven strategies—completely free.
Sign up for email alerts to get the moves before our social media followers.
Read Next: This Small Wearable Tech Stock Just Joined Wall Street’s Crypto Treasury Movement
Join the Discussion in the WVC Facebook Investor Group
Do you have a stock tip or news story suggestion? Please email us at: invest@wealthyvc.com.
Disclaimer: Wealthy VC does not hold a position in any of the stocks, ETFs or cryptocurrencies mentioned in this article.