Profusa Charts Bold Path to $250 Million Revenue by 2030
Digital health pioneer leverages its tissue-integrated biosensor platform, projecting a sequential launch of its Lumee™ oxygen and glucose monitors to capture a vast, untapped market.

Digital health company Profusa (NASDAQ: PFSA) recently laid out one of the most ambitious commercial roadmaps the biosensor sector has seen in years. The Berkeley-based firm is projecting a steep revenue ramp that it believes could culminate in $200 to $250 million in annual revenue by 2030.
This is not a far-off dream based on a single product. Profusa’s management has detailed a clear, step-by-step strategy, starting with the impending European launch of its Lumee™ tissue oxygen monitoring system in the second quarter of 2026. This initial launch will serve as the foundation for a much larger platform, which includes a highly anticipated continuous glucose monitor designed to serve a market of over 500 million people.
The company is moving from a decade of intensive research and development into a full-scale commercial operation. With manufacturing capabilities secured, key distribution channels materializing, and influential clinical leaders already signing on, Profusa is signaling to investors that its execution phase has officially begun.
A First-of-its-Kind Platform
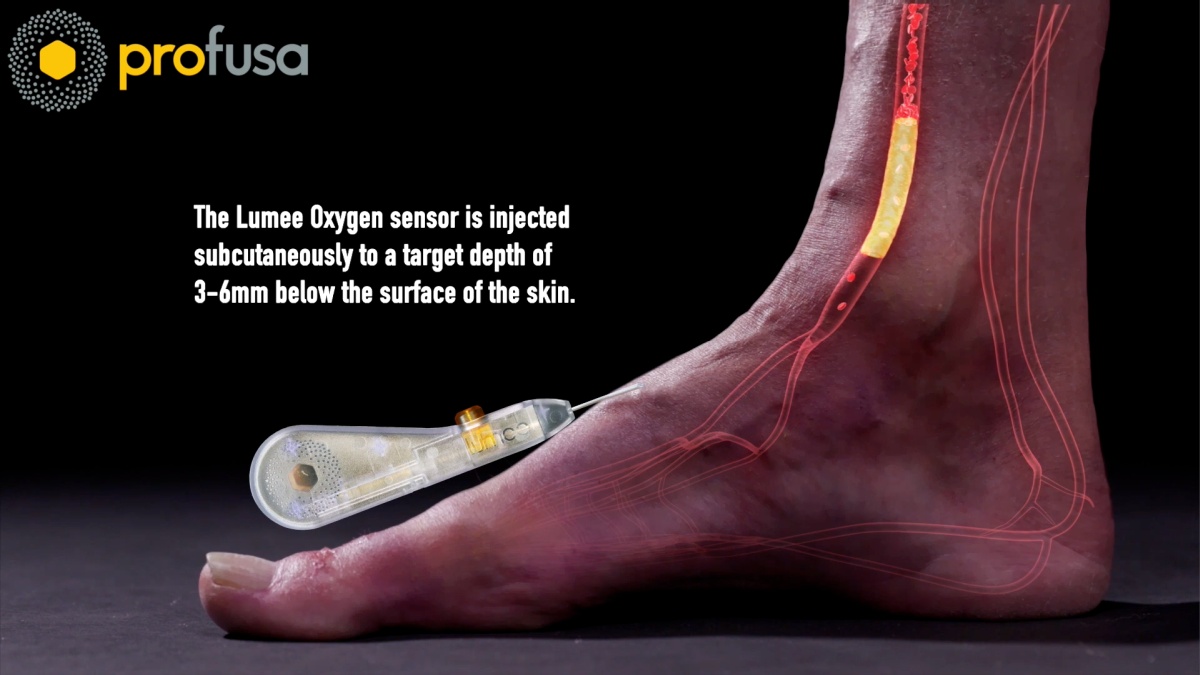
Profusa’s entire strategy hinges on its unique, tissue-integrated biosensor technology. This isn’t another wearable strap or surface-level patch. The Lumee platform uses a tiny, flexible biosensor injected just under the skin. This sensor, which can last for years, continuously measures an individual’s body chemistry. A detachable smart patch worn on the skin’s surface reads the sensor’s fluorescent signal and sends the real-time data to a smartphone.
This approach bypasses many of the limitations of current monitoring devices, offering a continuous stream of medical-grade biochemical data. The company believes this will revolutionize patient care, moving it from the clinic to the home.
Profusa’s Chairman and CEO, Ben Hwang, Ph.D., emphasized the disruptive nature of this technology, built on a robust foundation, stating:
“We believe Profusa is bringing to market a first-of-its-kind, disruptive biochemistry monitoring platform designed for use both in the clinic and at home. Backed by more than a decade of development and over $100 million in investment, our Lumee™ technology enables continuous, real-time measurement of tissue oxygen directly within the body—offering a less invasive, more convenient experience for patients while reducing costs for healthcare systems and insurers through earlier detection and improved disease management.”
The market opportunity Profusa targets is massive. Dr. Hwang estimates a $10.5 billion global addressable market for tissue oxygen monitoring alone, spanning peripheral artery disease (PAD), chronic wounds, and critical limb ischemia (CLI). The follow-on glucose platform targets an even larger prize: the 500 million people worldwide living with type 1, type 2, or pre-diabetes, a population vastly underserved by current continuous glucose monitors (CGMs) that primarily focus on 9 million type 1 patients.
The Financial Roadmap to 2030
Profusa’s CFO, Fred Knechtel, provided a detailed financial breakdown of this aggressive growth plan. The revenue stream starts small and then builds rapidly as new products and geographies come online.
The company projects $0.5 to $2 million in revenue in 2026, driven entirely by the Lumee oxygen system’s Q2 launch in the European Union. Profusa anticipates EU CE marking certification late in the first quarter of 2026, paving the way for the commercial rollout. Revenue models project €600 to €800 per procedure, with equipment sales adding another €30,000 to €40,000 per center annually.
From there, the growth accelerates. “We expect to layer on additional indications on our Lumee platform technology over time,” Mr. Knechtel stated. “In 2027, we plan to enter the US tissue oxygen monitoring market in the first quarter and add continuous glucose monitoring in the EU in mid-2027.”
The dual launch of U.S. oxygen and EU glucose is expected to catapult revenue to between $9 and $13 million in 2027.
This multi-pronged approach builds toward the 2030 target of $200 to $250 million. The company expects this revenue to be fairly evenly split, with $100 to $120 million coming from the expanded US/EU oxygen market and $100 to $130 million coming from the glucose monitor and other potential indications such as lactate, carbon dioxide, and sodium.
“We look forward to executing on this aggressive and dynamic growth agenda,” Mr. Knechtel concluded.
Also Read: Why Big Investors Who Can’t Buy Crypto Are Gobbling Up These Stocks Instead
De-Risking the Launch: Manufacturing and Distribution
A plan this ambitious requires flawless execution, and Profusa has spent the last several months actively shoring up its supply chain and commercial footprint.
On the manufacturing front, the company recently announced the completion of its manufacturing build-out. Its new Controlled Environment Room (CER) completed its first sensor production run in October. Critically, Profusa reports that its production capacity for sensors, pens, and patch/readers is already more than double what is required to meet its 2026 revenue targets. This surplus capacity provides a significant buffer against initial supply chain hiccups. Product shipments to its new European distributors are on track to begin in the first quarter of 2026.
Simultaneously, Profusa has been securing the very distribution channels that will receive those first shipments. The company recently signed a letter of intent with Angiopro GmbH, a specialized MedTech distributor. This partnership will cover initial commercialization in key markets including Germany, the Benelux countries, Austria, the United Kingdom, and Scandinavia.
This deal, combined with a previously announced distributor in Spain, means Profusa has already secured sales coverage for approximately 35% of the European Union population. This groundwork makes the 2026 and 2027 European revenue targets appear well within reach.
Winning Clinical Buy-In
The final, and perhaps most critical, piece of the puzzle is clinical adoption. A new technology is worthless if doctors do not trust it. Here, Profusa is building powerful momentum by collaborating directly with key opinion leaders in the vascular field.
In France, the company has established a collaboration with Pr. Yann Gouëffic, a leading surgeon in the field of critical limb-threatening ischemia (CLTI). Pr. Gouëffic and his FRANCE-PAD network of hospitals account for approximately 8% of all CLTI cases in the nation. He will not only adopt the Lumee technology in his practice but also in clinical studies to advance its use for home monitoring.
Pr. Yann Gouëffic commented:
“My practice, in addition to those of my affiliates, treats a meaningful number of CLTI cases in France and I believe Profusa’s Lumee technology to be a game changer in our patient care program and a true advancement clinical disease governance.”
This endorsement is bolstered by a similar collaboration in Austria with Prof. Dr. Marianne Brodmann at the Medical University of Graz. Prof. Brodmann is an ideal partner, as she served as the principal investigator for the very clinical studies that supported the Lumee oxygen CE mark. Her deep familiarity with the technology makes her decision to adopt it in her practice, which handles approximately 1,500 vascular procedures annually, a powerful validation for the entire clinical community.
With manufacturing secured, distribution channels established, and influential surgeons already on board, Profusa has transformed its 2030 vision from a simple projection into a credible, multi-stage execution plan. All eyes will now be on Europe for the Q2 2026 launch, the first of many catalysts on the company’s ambitious path.
Read Next: Why Nvidia’s Tech Investments Could Transform the Entire AI Innovation Ecosystem
Join the Discussion in the WVC Facebook Investor Group
Do you have a stock tip or news story suggestion? Please email us at: invest@wealthyvc.com.
Wealthy VC and its employees are not Registered Investment Advisors, Broker-Dealers or a member of any association for other research providers in any jurisdiction whatsoever and we are not qualified to give financial advice. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. Wealthy VC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled through their website, news releases, and corporate filings, or is available from public sources and Wealthy VC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. Our website and newsletter are for entertainment purposes only. This website is NOT a source of unbiased information. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment.
Release of Liability: Through the use of this email and/or website advertisement, by viewing or using it, you agree to hold Wealthy VC, its operators, owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. Wealthy VC-sponsored advertisements do not purport to provide an analysis of any company’s financial position, operations or prospects and this is not to be construed as a recommendation by Wealthy VC or an offer or solicitation to buy or sell any security.
None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provided herein. Instead, Wealthy VC strongly urges you to conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. This report/release/profile is a commercial advertisement and is for general information purposes only. We are engaged in the business of marketing and advertising companies for monetary compensation unless otherwise stated below. Profusa, Inc. (NASDAQ: PFSA) has compensated wealthyvc.com’s parent company, 1000358139 Ontario Limited, USD $568,000 by wire transfer. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D and all reports published on SEDAR if the company featured is Canadian. Wealthy VC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors with a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact and may be “forward-looking statements”. Forward-looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward-looking statements in this action may be identified through the use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions “may”, “could”, or “might” occur. Understand there is no guarantee past performance will be indicative of future results. Past Performance is based on the security’s previous day’s closing price and the high of-day price during our promotional coverage.
In preparing this publication, Wealthy VC has relied upon information supplied by various public sources and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this email and website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this email and website are believed to be reliable, however, Wealthy VC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of material facts from such advertisement. Wealthy VC is not responsible for any claims made by the companies advertised herein, nor is Wealthy VC responsible for any other promotional firm, its program or its structure.